 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| FGC-H5225 | Human | Human FGF R2 (IIIc) protein, His Tag (MALS verified) |  |

|
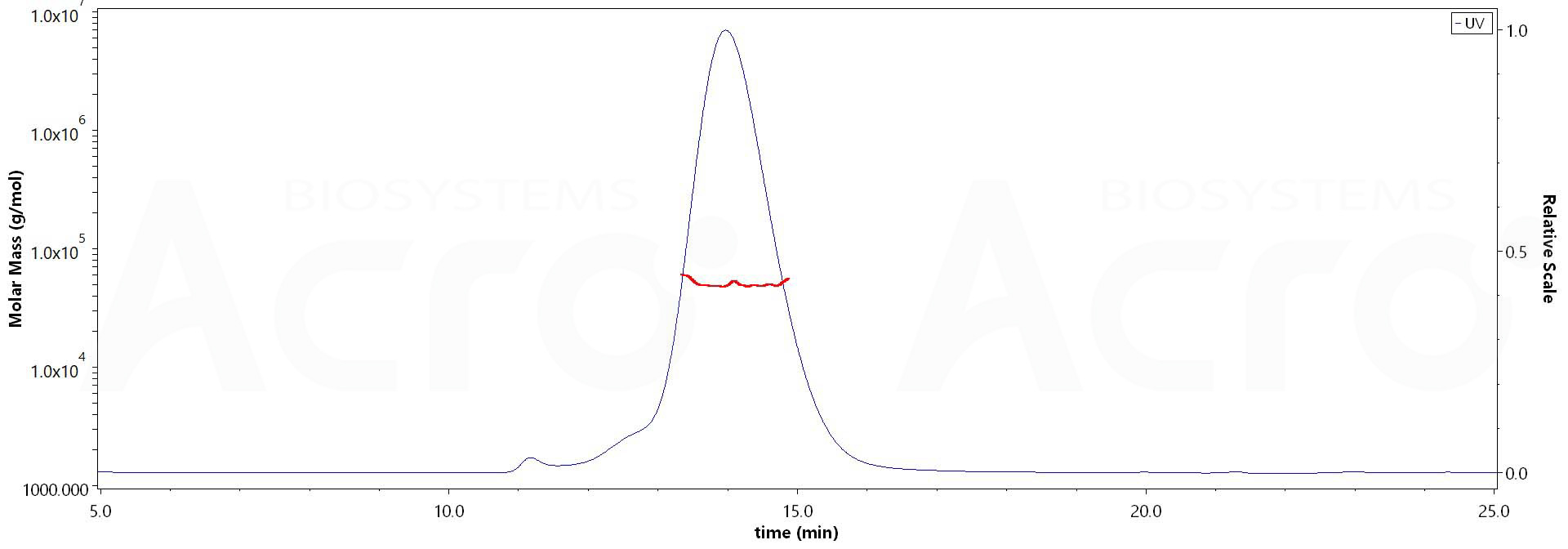
The purity of Human FGFR2 (IIIc), His Tag (Cat. No. FGC-H5225) is more than 90% and the molecular weight of this protein is around 45-55 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Infigratinib | BGJ-398; NVP-BGJ398 | Approved | Novartis Pharma Ag | TRUSELTIQ | United States | Cholangiocarcinoma | Helsinn Healthcare Sa | 2021-05-28 | Nasopharyngeal Neoplasms; Uterine Cervical Neoplasms; Adenocarcinoma; Glioma; Carcinoma, Pancreatic Ductal; Carcinoma, Squamous Cell; Gastrointestinal Stromal Tumors; Oropharyngeal Neoplasms; Cholangiocarcinoma; Central Nervous System Neoplasms; Urinary Bladder Neoplasms; Colonic Neoplasms; Biliary Tract Neoplasms; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Achondroplasia; Papillomavirus Infections; Neoplasms; Squamous Cell Carcinoma of Head and Neck; Rectal Neoplasms; Esophageal Neoplasms; Stomach Neoplasms; Hematologic Neoplasms; Solid tumours | Details |
| Futibatinib | TAS-120 | Approved | Taiho Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd | LYTGOBI | United States | Cholangiocarcinoma | Taiho Oncology Inc | 2022-09-30 | Solid tumours; Bone Marrow Neoplasms; Stomach Neoplasms; Carcinoma; Esophageal Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Central Nervous System Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Sarcoma; Lymphoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | EU | systemic sclerosis-associated interstitial lung disease | Boehringer Ingelheim International Gmbh | 2014-10-15 | Gliosarcoma; Breast Neoplasms; Sarcoma; systemic sclerosis-associated interstitial lung disease; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Genital Neoplasms, Female; Silicosis; Prostatic Neoplasms; Carcinoma, Squamous Cell; Appendiceal Neoplasms; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Carcinoma, Hepatocellular; Colonic Neoplasms; Ovarian Neoplasms; Telangiectasia, Hereditary Hemorrhagic; Esophageal Neoplasms; Rejection of lung transplantation; Carcinoid Tumor; Endometrial Stromal Tumors; Carcinoma, Renal Cell; Radiation Pneumonitis; Neoplasms; Idiopathic Pulmonary Fibrosis; Solid tumours; Scleroderma, Systemic; Glioblastoma; Small Cell Lung Carcinoma; Mesothelioma; Neuroendocrine Tumors; Pulmonary Fibrosis; Lung Diseases, Interstitial; Multiple Myeloma; Asbestosis; Oligodendroglioma | Details |
| Lenvatinib Mesylate | ER-203492-00; E-7080; MK-7902 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | Japan | Carcinoma, Renal Cell | Merck Sharp & Dohme Corp | 2015-02-13 | Liver Diseases; Paraganglioma; Neoplasm Metastasis; Thyroid Cancer, Papillary; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Osteosarcoma; Cholangiocarcinoma; Breast Neoplasms; Solid tumours; Neuroendocrine Tumors; Carcinoma, Adenoid Cystic; Adenocarcinoma, Follicular; Kidney Diseases; Neoplasms; Adenocarcinoma of Lung; Thyroid Carcinoma, Anaplastic; Pheochromocytoma; Renal Insufficiency; Esophageal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | Mainland China | Gastrointestinal Stromal Tumors | Bayer Pharma Ag | 2012-09-27 | Leukemia, Myeloid, Acute; Sarcoma; Gastrinoma; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Bile Duct Neoplasms; Fallopian Tube Neoplasms; Esophageal adenocarcinoma; Adenoma; Thyroid Neoplasms; Lung Neoplasms; Thymoma; Carcinoma, Hepatocellular; Adenocarcinoma; Melanoma; Neoplasm Metastasis; Gastrointestinal Neoplasms; Somatostatinoma; Carcinoma, Islet Cell; Ovarian Neoplasms; Liver Neoplasms; Hemangiosarcoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Carcinoid Tumor; Insulinoma; Solid tumours; Colonic Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Glioblastoma; Carcinoma, Transitional Cell; Carcinoma, Adenoid Cystic; Glucagonoma | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Thyroid Neoplasms | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Esophageal Squamous Cell Carcinoma; Bone Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Hepatic Insufficiency; Gastrointestinal Stromal Tumors; Urologic Neoplasms; Sarcoma, Alveolar Soft Part; Endometrial Neoplasms; Gallbladder Neoplasms; Bile Duct Diseases; Thyroid Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Osteoma; Small Cell Lung Carcinoma; Head and Neck Neoplasms; Solid tumours; Biliary Tract Neoplasms; Drug-Related Side Effects and Adverse Reactions; Ovarian Neoplasms; Esophageal Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Leiomyosarcoma; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Carcinoma, Ovarian Epithelial; Sarcoma, Synovial; Neuroendocrine Tumors; Liver Diseases; Sarcoma; Medullary thyroid cancer (MTC); Nasopharyngeal Carcinoma | Details |
| Erdafitinib | 890E37NHMV; JNJ-42756493; G-024; JNJ-493 | Approved | Astex Pharmaceuticals Inc | Balversa | South Korea | Carcinoma, Transitional Cell | Janssen Biotech Inc | 2019-04-12 | Neoplasms, Neuroepithelial; Osteosarcoma; Neuroblastoma; Breast Neoplasms; Urologic Neoplasms; Hepatic Insufficiency; Histiocytic Sarcoma; Bile Duct Neoplasms; Lymphoma; Lymphoma, Non-Hodgkin; Sarcoma; Glioma; Carcinoma, Squamous Cell; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Xanthogranuloma, Juvenile; Neoplasms, Germ Cell and Embryonal; Neuroectodermal Tumors, Primitive, Peripheral; Rhabdoid Tumor; Hematologic Neoplasms; Rhabdomyosarcoma; Ependymoma; Medulloblastoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma; Hepatoblastoma; Solid tumours; Neoplasms; Carcinoma, Transitional Cell; Wilms Tumor; Multiple Myeloma; Urinary Bladder Neoplasms; Central Nervous System Neoplasms; Histiocytosis, Langerhans-Cell; Sarcoma, Ewing | Details |
| Pemigatinib | INCB-054828; INCB-54828; IBI-375 | Approved | Incyte Corp | Pemazyre, 伯坦, 达伯坦 | Mainland China | Biliary Tract Neoplasms | Innovent Biologics(Suzhou) Co Ltd | 2020-04-17 | Translocation, Genetic; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Glioma; Lung Neoplasms; Endometrial Neoplasms; Bile Duct Neoplasms; Colitis, Ulcerative; Urologic Neoplasms; Colorectal Neoplasms; Breast Neoplasms; Cholangiocarcinoma; Solid tumours; Multiple Myeloma; Urinary Bladder Neoplasms; Glioblastoma; Carcinoma, Transitional Cell; Pancreatic Neoplasms; Myeloproliferative Disorders; Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Carcinoma; Biliary Tract Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| BPI-17509 | BPI-17509 | Phase 1 Clinical | Betta Pharmaceuticals Co Ltd | Solid tumours | Details |
| CPL-304110 | CPL-304110; CPL-304-110 | Celon Pharma Sa | Details | ||
| R-1530 | RG1530; R-1530; RG-1530 | F. Hoffmann-La Roche Ltd | Details | ||
| LY-2874455 | LY-2874455 | Eli Lilly And Company | Details | ||
| TT-00434 | TT-00434 | Phase 1 Clinical | TransThera Sciences (Nanjing) Inc | Solid tumours; Urinary Bladder Neoplasms | Details |
| Aprutumab ixadotin | BAY-1187982 | Bayer AG | Details | ||
| DS-1123 | DS-1123; DS-1123a | Daiichi Sankyo Co Ltd | Details | ||
| ODM-203 | ODM-203 | Orion Corp | Details | ||
| Aprutumab | FGFR-moAb; FGFR2-TTC; BAY-1179470 | Bayer AG | Details | ||
| SC-0011 | SC-0011 | Phase 1 Clinical | Shijiazhuang Zhikang Hongren New Drug Development Co Ltd | Solid tumours | Details |
| Efruxifermin | AKR-001; AMG-876; EFX | Phase 2 Clinical | Amgen Inc | Non-alcoholic Fatty Liver Disease; Diabetes Mellitus | Details |
| Derazantinib | ARQ-087.2HCl; AQ-14741087; ARQ-087; BAL-087 | Phase 2 Clinical | Arqule Inc | Solid tumours; Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Cholangiocarcinoma; Urogenital Neoplasms; Carcinoma, Hepatocellular | Details |
| Lucitanib | AL-3810; CO-3810; S-80881 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Carcinoma, Small Cell; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Zoligratinib | CH-5183284; FF-284; Debio-1347 | Phase 2 Clinical | Chugai Pharmaceutical Co Ltd | Solid tumours; Breast Neoplasms | Details |
| KIN-3248 | KIN-3248; KIN-003 | Phase 1 Clinical | Kinnate Biopharma Inc | Solid tumours; Carcinoma, Transitional Cell; Cholangiocarcinoma | Details |
| HH185 | 3-D185; HH-185; 3D185; 3D-185 | Phase 2 Clinical | Shanghai Medicilon Inc, ShangHai HaiHe Biopharma Co Ltd, Shanghai Institute Of Materia Medica, Chinese Academy Of Sciences | Solid tumours; Cholangiocarcinoma | Details |
| Gunagratinib | ICP-192 | Phase 2 Clinical | Beijing Tiancheng Pharmaceutical Technology Co Ltd | Solid tumours; Head and Neck Neoplasms; Biliary Tract Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Cholangiocarcinoma; Lung Neoplasms | Details |
| Rogaratinib | BAY-1163877 | Phase 2 Clinical | Bayer AG | Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Urinary Bladder Neoplasms; Sarcoma; Breast Neoplasms; Gastrointestinal Stromal Tumors; Carcinoma, Non-Small-Cell Lung | Details |
| RLY-4008 | RLY-4008 | Phase 2 Clinical | Relay Therapeutics Inc | Cholangiocarcinoma; Translocation, Genetic | Details |
| Tasurgratinib | E-7090 | Phase 2 Clinical | Eisai Co Ltd | Biliary Tract Neoplasms; Solid tumours; Neoplasms; Cholangiocarcinoma; Breast Neoplasms; Hepatic Insufficiency | Details |
| ABSK-061 | ABSK061 | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| Bemarituzumab | FPA-144 | Phase 3 Clinical | Five Prime Therapeutics Inc | Solid tumours; Stomach Neoplasms; Esophageal Neoplasms; Neoplasms; Carcinoma, Transitional Cell; Urinary Bladder Neoplasms; Carcinoma, Squamous Cell; Adenocarcinoma; Gastrointestinal Neoplasms | Details |
| Alofanib | RPT-835 | Phase 1 Clinical | Russian Pharmaceutical Technologies Llc | Stomach Neoplasms | Details |
| HMPL-453 | HMPL-453 | Phase 2 Clinical | Solid tumours; Biliary Tract Neoplasms; Neoplasms, Mesothelial; Mesothelioma; Cholangiocarcinoma; Bile Duct Neoplasms | Details | |
| ABSK-121 | ABSK121; ABSK121-NX | Phase 1 Clinical | ABbisko Therapeutics Co Ltd | Solid tumours | Details |
| AZD-4547 | AZD-4547; ABSK-091; AZD4547; ABSK091 | Phase 2 Clinical | Astrazeneca Plc | Uterine Neoplasms; Breast Neoplasms; Prostatic Neoplasms; Cholangiocarcinoma; Colorectal Neoplasms; Carcinoma, Squamous Cell; Esophageal adenocarcinoma; Endometrial Neoplasms; Lung Neoplasms; Multiple Myeloma; Thyroid Neoplasms; Lymphoma; Glioma; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Uterine Cervical Neoplasms; Melanoma; Foodborne Diseases; Squamous Cell Carcinoma of Head and Neck; Hematologic Neoplasms; Ovarian Neoplasms; Liver Neoplasms; Stomach Neoplasms; Gastrointestinal Diseases; Esophageal Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Solid tumours; Carcinoma, Transitional Cell; Neoplasms; Skin Neoplasms; Pancreatic Neoplasms; Carcinoma, Ovarian Epithelial; Colonic Neoplasms; Urinary Bladder Neoplasms | Details |
This web search service is supported by Google Inc.